Exploring Glucagon-like Peptide-1 receptor agonist (GLP-1 RA) Use in Patients with Cirrhosis
- Kimmy Nguyen
- Jul 2, 2025
- 4 min read
Written by Braylee Wardwell, PharmD and Marissa Salvo, PharmD, BCACP, FCPA, FCCP
What is Cirrhosis?
Cirrhosis is a condition where healthy liver tissue is replaced by scar tissue. As cirrhosis progresses, scar tissue can block blood flow to the liver and cause liver failure.(1) Leading causes of cirrhosis include hepatitis B and C infections, excessive alcohol consumption, and metabolic dysfunction due to diabetes, obesity, hypertension or lipid disorders. There are two stages of cirrhosis, compensated and decompensated. In compensated cirrhosis, patients are asymptomatic, and risk factors of cirrhosis as stated above are detected in routine blood work. In decompensated cirrhosis, patients are symptomatic, due to significant fibrotic changes, and present with one or more of the following: ascites, jaundice, hepatic encephalopathy, or variceal hemorrhage.(2) Of note cirrhosis is one of five conditions encompassed by the broad category metabolic dysfunction-associated steatotic liver disease (MASLD).(3)
Background: How GLP-1 RAs Work and Potential Cirrhosis Benefit
GLP-1 RAs stimulate prandial insulin secretion, limiting glucagon production, slowing gastric motility and increasing satiety. GLP-1 RAs are approved to treat disease states including diabetes, obesity, and obstructive sleep apnea. Treating metabolic risk factors for developing MASLD, such as diabetes and obesity, can decrease the risk of developing MASLD; or if already diagnosed, can slow progression to cirrhosis.(3)
Current Guidelines: GLP-1 RA Use in Cirrhosis
The guidelines for MASLD management, updated in 2024, are a collaborative effort between the European Association for the Study of the Liver, European Association for the Study of Diabetes, and European Association for the Study of Obesity. These guidelines state that GLP-1 RAs are safe to use in patients with compensated cirrhosis for approved indications of diabetes and obesity as use improves cardiometabolic outcomes. However, currently the use of GLP-1 RAs is not recommended as treatment of MASLD.(3)
The American Diabetes Association 2025 guidelines similarly state GLP-1 RAs are recommended in adults with diabetes, obesity, and MASLD in combination with diet and exercise for weight loss; or for patients with diabetes and at high risk of liver fibrosis, as there are potential beneficial effects on MASLD.(4)
Studies of GLP-1 RA Use in Patients with Cirrhosis
A retrospective study in Taiwan, using matched pairs in which half of the patients used GLP-1 RAs, and the other half did not, included patients with type 2 diabetes and compensated cirrhosis. Compared to non-users, GLP-1 RA users had a statistically significantly lower all-cause mortality, lower MACE, and lower development of decompensated cirrhosis.(5)
A retrospective cohort study in the United States included patients with MASLD and type 2 diabetes. Patients were initiated on either a GLP-1 RA or dipeptidyl peptidase 4 inhibitor (DPP-4i). Compared to DPP-4i users, GLP-1 RA users had statistically significant lower all-cause mortality, progression to cirrhosis, and cirrhosis-related complications.(6)
Another retrospective cohort study in the United States included patients with type 2 diabetes and cirrhosis. Patients were initiated on DPP-4i, sulfonylurea, SGLT-2i, or GLP-1 RA. Progression to decompensated cirrhosis in GLP-1 RA users was statistically significantly lower than in DPP-4i users and sulfonylurea users. There was no statistical significance noted in comparing GLP-1 RA users to SGLT-2i users.(7)
Table 1: Comparison of Studies Assessing GLP-1 RA Use
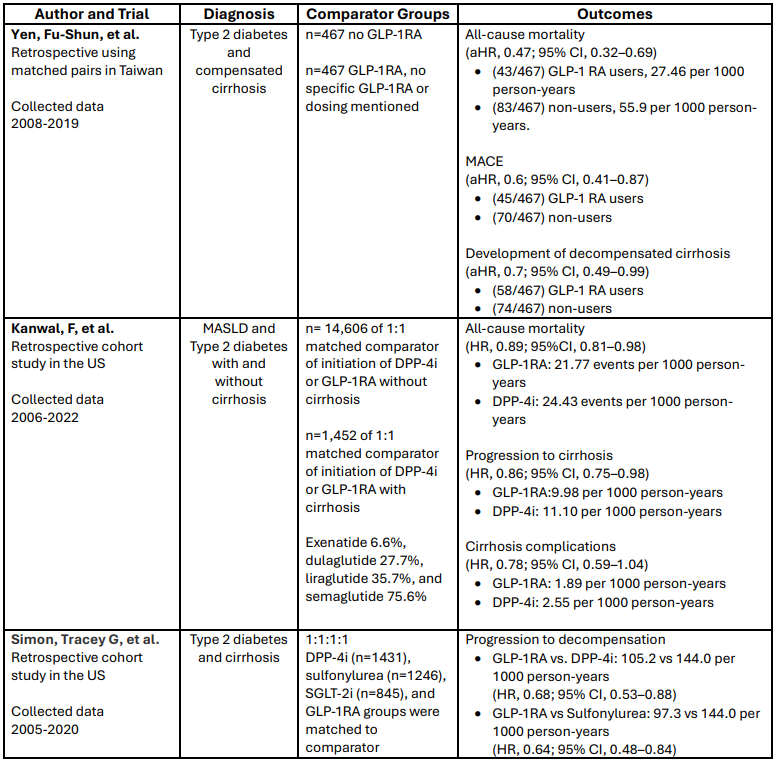
Abbreviations: aHR, adjusted hazard ratio; DPP-4i, dipeptidyl peptidase 4 inhibitor; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HR, hazard ratio; MACE, major adverse cardiovascular events; MASLD, metabolic dysfunction-associated steatotic liver disease; SGLT-2i, sodium-glucose cotransporter-2 inhibitor
Conclusion
In conclusion, there are no randomized controlled trials studying the use of GLP-1 RAs as a treatment of cirrhosis, and current data supporting the use comes from retrospective studies. Current literature supports the use of GLP-1 RAs for patients with compensated cirrhosis to prevent or slow progression to decompensated cirrhosis. GLP-1 RA use may also benefit patients who have metabolic dysfunction in preventing or slowing progression to cirrhosis, and its use in patients with cirrhosis has been associated with a decrease in all-cause mortality.
1. National Institute of Diabetes and Digestive and Kidney Diseases. Cirrhosis | NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases. Published June 10, 2019. https://www.niddk.nih.gov/health-information/liver-disease/cirrhosis.
2. Stages of Cirrhosis - Viral Hepatitis and Liver Disease. www.hepatitis.va.gov. https://www.hepatitis.va.gov/cirrhosis/background/stages.asp.
3. EASL–EASD–EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD) Tacke, Frank et al. Journal of Hepatology, Volume 81, Issue 3, 492-542.
4. American Diabetes Association Professional Practice Committee; 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2025. Diabetes Care 1 January 2025; 48 (Supplement_1): S181–S206. https://doi.org/10.2337/dc25-S009.
5. Glucagon-like Peptide-1 Receptor Agonist Use in Patients With Liver Cirrhosis and Type 2 Diabetes. Yen, Fu-Shun et al. Clinical Gastroenterology and Hepatology, Volume 22, Issue 6, 1255 - 1264.e18.
6. Kanwal F, Kramer JR, Li L, et al. GLP-1 Receptor Agonists and Risk for Cirrhosis and Related Complications in Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease. JAMA Intern Med. 2024;184(11):1314-1323. doi:10.1001/jamainternmed.2024.4661.
7. Glucagon-Like Peptide-1 Receptor Agonists and Hepatic Decompensation Events in Patients With Cirrhosis and Diabetes. Simon, Tracey G. et al. Clinical Gastroenterology and Hepatology, 2022 Jun; Volume 20, Issue 6, 1382-1393.e19.

Braylee Wardwell, PharmD
PGY2 Ambulatory Care Resident
UConn Health
Farmington, CT

Marissa Salvo, PharmD, BCACP, FCPA, FCCP
Associate Clinical Professor of Pharmacy Practice
University of Connecticut School of Pharmacy
Storrs, CT



Comments